About ACCRUFeR
ACCRUFeR® (ferric maltol) is an FDA approved oral iron treatment for adults and children 10 years of age and older with iron deficiency.2
Uniquely formulated with a MALTOL SHIELDTM
ACCRUFeR is a stable complex of ferric iron (Fe3+) and maltol, a naturally occurring sugar derivative.2 Unlike iron salts, this iron-sugar derivative complex stays intact in the stomach, dissociating when it reaches the duodenum for optimal iron absorption.2
The ACCRUFeR Mechanism of Action2-4
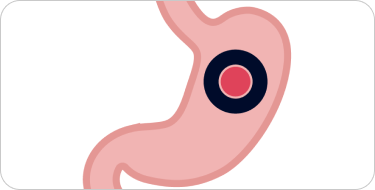
Tightly bound in the stomach
The MALTOL SHIELDTM protects iron from the stomach, remaining tightly bound as it passes through.
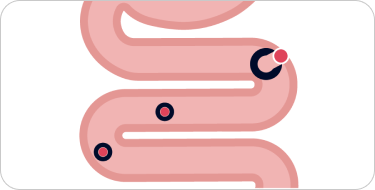
Dissociates upon uptake in the duodenum
Iron remains bioavailable, chelated, and ready to replenish iron stores. Excess iron is excreted in the stool.
Why the MALTOL SHIELDTM matters:3,4,6
- It allows iron in ACCRUFeR to remain bioavailable until it reaches the duodenum, where it can be efficiently absorbed.
- It reduces the likelihood of reactive oxygen species (ROS) formation, minimizing the risk for GI side effects and irritation or damage to the intestinal lining.
- Since the iron and maltol remain chelated until absorption, there is less free iron in the gut, lessening the risk for added bowel inflammation.
Intolerable GI side effects can be a barrier to staying on oral iron replacement therapy.
The safety and efficacy profile in ACCRUFeR’s pediatrics study was consistent with that observed in adults with iron deficiency or iron deficiency anemia.2
Why ACCRUFeR?
Free Samples of ACCRUFeR
Your patients deserve a tolerable oral iron treatment. Put ACCRUFeR to the test with free 5-day samples for your patients through MySampleCloset.
Request SamplesReferences:
1. Schmidt C, Ahmad T, Tulassay Z, et al. Ferric maltol therapy for iron deficiency anaemia in patients with inflammatory bowel disease: long-term extension data from a phase 3 study. Aliment Pharmacol Ther. 2016;44(3):259-270. doi:10.1111/apt.13665
2. ACCRUFeR® full prescribing information. Shield Therapeutics, 2025.
3. Stallmach A, Büning C. Ferric maltol (ST10): a novel oral iron supplement for the treatment of iron deficiency anemia in inflammatory bowel disease. Expert Opin Pharmacother. 2015;16:2859-2867.
4. European Medicines Agency. Accessed March 17, 2021. https://www.ema.europa.eu/en/documents/variation-report/feraccru-h-c-2733-ii-0010-epar-assessment-report-variation_en.pdf
5. Gasche C, Ahmad T, Tulassay Z, et al. Ferric maltol is effective in correcting iron deficiency anemia in patients with inflammatory bowel disease: results from a phase-3 clinical trial program. Inflamm Bowel Dis. 2015;21(3):579-588. doi:10.1097/mib.0000000000000314
6. Pergola PE, Kopyt NP. Oral ferric maltol for the treatment of iron-deficiency anemia in patients with CKD: a randomized trial and open-label extension. Am J Kidney Dis. 2021;78(6):846-856.e1. doi:10.1053/j.ajkd.2021.03.020
7. Data on file. Shield Therapeutics Inc. 2019
29. Lindgren S, Wikman O, Befrits R, et al. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: a randomized, controlled, evaluator-blind, multicentre study. Scand J Gastroenterol. 2009;44(7):838-845. doi:10.1080/00365520902839667
