Established Safety and Efficacy
ACCRUFeR® (ferric maltol) is backed by a robust clinical profile
The safety and efficacy of ACCRUFeR for the treatment of iron deficiency anemia were established in three randomized, double-blind, placebo-controlled trials.1,2,5
ACCRUFeR increased and maintained Hb, ferritin, and TSAT across three clinical studies
ACCRUFeR was studied in two trials with patients with inflammatory bowel disease (IBD) and one trial with patients with stage 3 or 4 chronic kidney disease (CKD). Both the IBD and CKD studies had an open-label extension, providing safety and efficacy data for up to 64 weeks.2,6 No patients treated with ACCRUFeR in the long-term studies required IV iron intervention.1,2
Free Samples of ACCRUFeR
Your patients deserve a tolerable oral iron treatment. Put ACCRUFeR to the test with free 5-day samples for your patients through MySampleCloset.
Request SamplesIBD studies:
Patients with inflammatory bowel disease saw significant and rapid improvements in iron indices
Patients in the trial had a confirmed diagnosis of mild-to-moderate ulcerative colitis (SCCAI <4) or Crohn’s disease (CDAI <220); hemoglobin levels between ≥9.5 g/dL and <12.0 g/dL for females and ≥9.5 g/dL and <13.0 g/dL for males1,5; serum ferritin levels of <30 μg/L at screening; and previous failure to treat their iron deficiency with a ferrous salt because of the lack of tolerability or efficacy.3,5
LS Mean (SE) Hb concentration from baseline to Week 12:1,5
Primary endpoint: The LS mean (SE) improvement in Hb in the ACCRUFeR group was 2.25 (0.12) g/dL (one-sided 97% CI, 1.81; P<.0001 based on ANCOVA).2
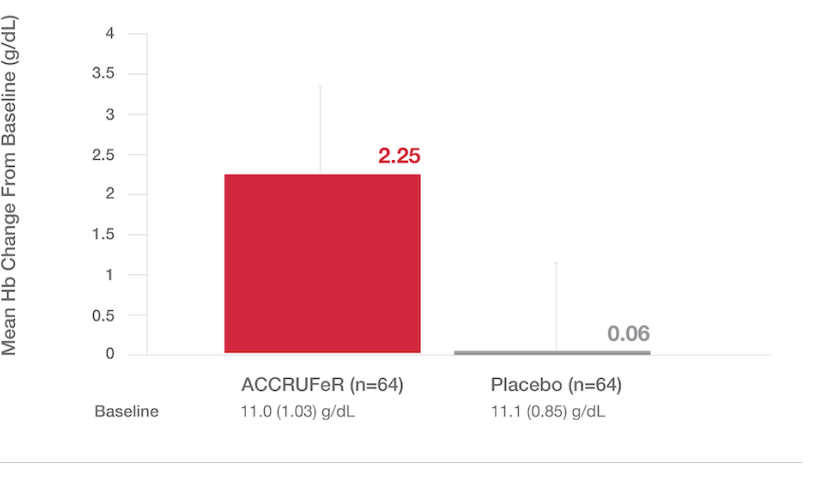
- A ≥1 g/dL change in Hb at week 12 was observed in 78% of patients taking ACCRUFeR (n=64) vs. 11% of patients in the placebo group (n=64).*
- Statistical significance was achieved as early as week 4.2
*Based on responder analysis
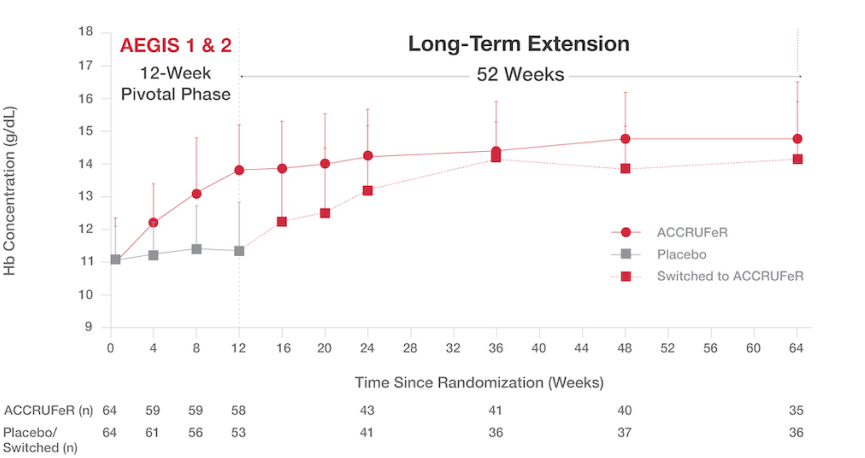
Increased Hb concentrations were maintained over 64 weeks.
- The mean improvement in Hb concentration for patients taking ACCRUFeR was 3.07 (1.46) g/dL from baseline to Week 64.¹
- The cumulative proportion of patients who maintained normal Hb was 71% at Week 12, 79.7% at Week 24, and 86.1% at Week 64.¹
Absolute Hb concentrations from baseline to Week 645
ACCRUFeR: Mean improvement in Hb concentration was 3.07 (1.46) g/dL from baseline to Week 64.
PLACEBO/SWITCHED: Mean improvement in Hb concentration was 2.19 (1.61) g/dL from baseline to Week 64.
ACCRUFeR increased ferritin levels by 17.3 mcg/L and TSAT by 18%2
| Ferritin and TSAT levels | ACCRUFeR | Placebo |
|---|---|---|
| Ferritin | 17.3 (28.30) mcg/L5 | 1.2 (7.85) mcg/L5 |
| TSAT | +18% (20.2)5 | -0.4% (7.8)5 |
- 128 IDA patients with inflammatory bowel disease (64 from each of two studies — 58 with ulcerative colitis, 70 with Crohn’s disease) were randomized 1:1.
- Hb concentrations were between ≥9.5 g/dL and <12g/dL for females and <13 g/dL for males and ferritin was <30 µg/L for all patients.
- All patients had previously failed on treatment with oral ferrous replacement products.¹
- A responder analysis was done defining treatment responders as patients who achieved increases in Hb of ≥1 g/dL or ≥2 g/dL, or Hb normalization by Week 12. Normalization of Hb was defined as Hb values ≥12 g/dL for females and ≥13 g/dL for males.
Primary endpoint: Mean difference in Hb concentration from baseline to Week 12 (one-sided 97% CI, 1.81; P<.0001 based on ANCOVA).
Secondary endpoints:
- Changes in Hb concentration from baseline to Weeks 4 and 8 and at Week 52
- Proportion of patients achieving normal Hb levels by Week 52
- Serum ferritin concentration from baseline through Week 52
- Percentage TSAT from baseline to Weeks 4 and 8
- Mean age: 38.5 (placebo) versus 40.1 (ACCRUFeR)
- Gender and race: 45 males and 83 females; 122 White and 6 unspecified other
CKD study:
Significant improvements were seen across Hb, ferritin, and TSAT in patients with stage 3 or 4 chronic kidney disease2,6
Patients in the study had either stage 3 or 4 CKD; hemoglobin levels between ≥8.0 g/dL and <11.0 g/dL for females and serum ferritin levels less than 250 μg/L with TSAT less than 25% or serum ferritin levels less than 500 μg/L with TSAT less than 15% at screening.
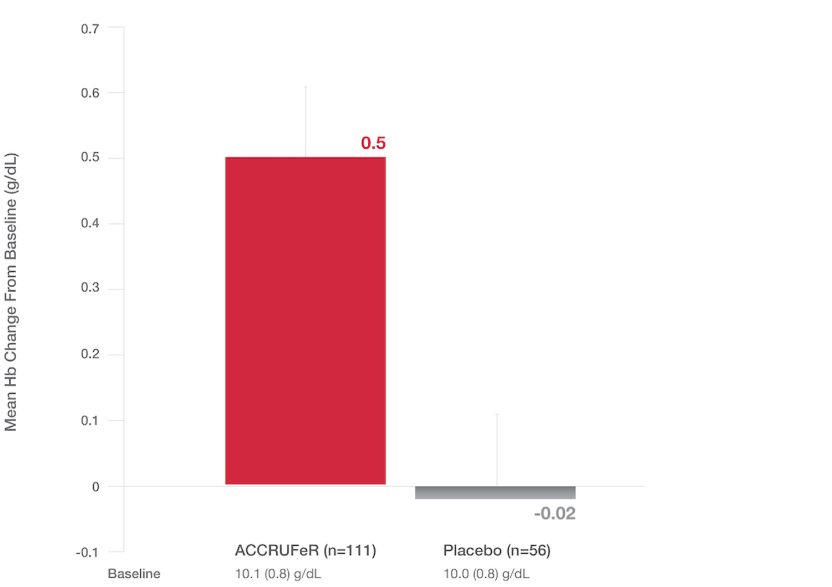
Primary endpoint: LS Mean (SE) change in Hb at Week 16
- The LS mean improvement in Hb in the ACCRUFeR group vs. placebo was 0.52 g/dL (0.21) (CI: 0.10, 0.93; P=0.0149).
- ACCRUFeR had an LS mean difference in change from baseline Hb of 0.13 g/dL at Week 4 and 0.46 g/dL at Week 8 vs. placebo.2
- Increases in Hb for patients taking ACCRUFeR at Weeks 4, 8, and 16 were consistent with changes seen in the AEGIS-IBD studies.5,6
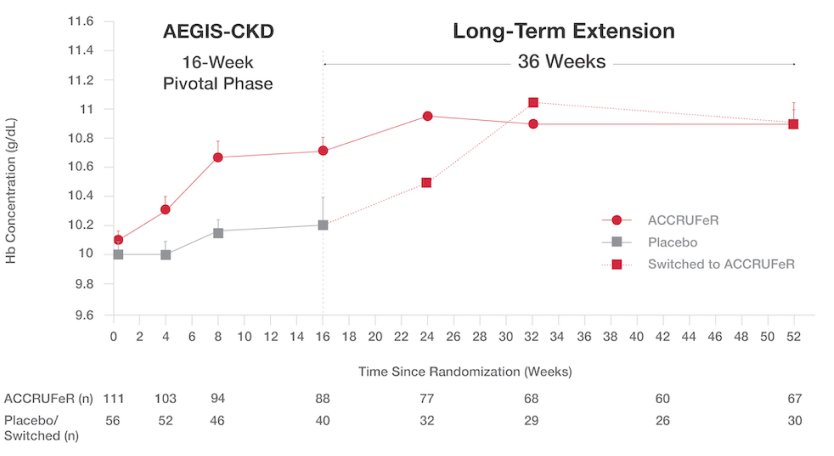
Patients with CKD saw improvements in Hb at the end of the initial 16-week study period and continued to improve during the 36-week open-label extension6
Absolute mean (SD) Hb concentrations from baseline (Day 0) to Week 52:6
- After Week 16, patients on placebo switched to treatment with ACCRUFeR. Patients already on ACCRUFeR maintained treatment for an additional 36 weeks.
- ACCRUFeR: Mean improvement in Hb concentration was 0.7 (1.7) g/dL from baseline to Week 52.
- Placebo/switched: Mean improvement in Hb concentration was 0.5 (1.4) g/dL from baseline to Week 52.
ACCRUFeR increased ferritin levels and TSAT2,6
- The mean change in ferritin concentration from baseline to Week 16 was 49.3 mcg/L for the ACCRUFeR group and 6.3 mcg/L for the placebo group, with a mean difference between the groups of 43.0 mcg/L (P<0.001).2,6
- The mean percentage change in TSAT from baseline (LS mean, SE) to Week 16 was 3.8 (0.6) for the ACCRUFeR group and -0.9 (0.9) for the placebo group, with a mean difference of 4.6 (1.1) (P<0.0001).2,6
- 167 stage 3 or 4 CKD patients with IDA were randomized 2:1 (ACCRUFeR to placebo).7
- Patients in the study had either stage 3 or 4 CKD; hemoglobin levels between ≥ 8.0 g/dL and < 11 g/dL and serum ferritin levels less than 250 μg/L with TSAT less than 25% or serum ferritin levels < 500 μg/Lwith TSAT less than 15% at screening.
- Primary endpoint: Change in Hb from baseline to Week 16.
- Secondary endpoints: The proportion of patients with hemoglobin increases of at least 1 g/dL and at least 2 g/dL at week 16; the proportion of patients achieving a hemoglobin concentration of at least 11.0 g/dL at week 16; change in hemoglobin concentration from baseline to weeks 4 and 8; and changes in ferritin, TSAT, and serum iron measures at weeks 4, 8, and 16.
- Mean age: 65.2 (placebo) versus 68.5 (ACCRUFeR); full age range of 30–90 years
- Gender and race: 50 males and 117 females; 123 White, 35 Black, and 9 other
4.6% of patients (n=175) discontinued ACCRUFeR because of adverse reactions compared to 2.5% of patients (n=120) on placebo.2,5,6
ACCRUFeR’s clinical trials established safety and unprecedented tolerability1
Adverse reactions that were reported by ≥1% of patients treated with ACCRUFeR during double-blind phases of the placebo-controlled period of pooled studies (AEGIS IBD and AEGIS CKD)*,2
Because ACCRUFeR’s MALTOL SHIELDTM keeps iron intact in the stomach, reactive oxygen species are less likely to form, which reduces the risk for GI upset.2,3 However, patients taking ACCRUFeR may still experience side effects, such as flatulence, diarrhea, or constipation.2
*Excluding the open-label extension period.
| Gastrointestinal Adverse Reaction | ACCRUFeR | Placebo |
|---|---|---|
| Flatulence | 4.6% | 0% |
| Diarrhea | 4% | 1.7% |
| Constipation | 4% | 0.8% |
| Discolored feces | 4% | 0.8% |
| Abdominal pain | 2.9% | 2.5% |
| Nausea | 1.7% | 0.8% |
| Vomiting | 1.7% | 0% |
| Abdominal discomfort | 1.1% | 0% |
| Abdominal distention | 1.1% | 0% |
References:
1. Schmidt C, Ahmad T, Tulassay Z, et al. Ferric maltol therapy for iron deficiency anaemia in patients with inflammatory bowel disease: long-term extension data from a phase 3 study. Aliment Pharmacol Ther. 2016;44(3):259-270. doi:10.1111/apt.13665
2. ACCRUFeR® full prescribing information. Shield Therapeutics, 2025.
3. Stallmach A, Büning C. Ferric maltol (ST10): a novel oral iron supplement for the treatment of iron deficiency anemia in inflammatory bowel disease. Expert Opin Pharmacother. 2015;16(18):2859-2867. doi:10.1517/14656566.2015.1096929
5. Gasche C, Ahmad T, Tulassay Z, et al. Ferric maltol is effective in correcting iron deficiency anemia in patients with inflammatory bowel disease: results from a phase-3 clinical trial program. Inflamm Bowel Dis. 2015;21(3):579-588. doi:10.1097/mib.0000000000000314
6. Pergola PE, Kopyt NP. Oral ferric maltol for the treatment of iron-deficiency anemia in patients with CKD: a randomized trial and open-label extension. Am J Kidney Dis. 2021;78(6):846-856.e1. doi:10.1053/j.ajkd.2021.03.020
7. Data on file. Shield Therapeutics Inc. 2019
